Chronic Kidney Disease in 2025: From Silent Epidemic to Treatable Risk State
- Andrew Kowalski
- Nov 30, 2025
- 7 min read
Updated: Jan 28
Andrew Kowalski, MD, FASN
Understanding Chronic Kidney Disease: A Growing Concern
Chronic Kidney Disease (CKD) has emerged as a significant challenge in global health. Current estimates indicate that approximately 850 million people worldwide live with CKD, with around 4 million receiving dialysis or living with a kidney transplant (Herrington et al., 2025). These numbers are staggering. What makes them alarming is the trajectory: CKD is projected to become the fifth leading underlying cause of death worldwide by 2050 (Herrington et al., 2025). The aging population, rising diabetes and hypertension diagnoses, and increasing obesity rates are pushing CKD higher on the mortality list, surpassing many cancers.

For much of its natural history, CKD remains asymptomatic. It can only be detected through laboratory testing, making it invisible to patients. This combination of high prevalence, silent progression, and potentially catastrophic outcomes makes CKD one of the most critical yet underutilized areas in population health management.
Unfortunately, primary health providers are struggling to maintain adequate numbers to see a reasonable amount of patients. Many of these providers fall victim to the system of "lab flagging." If a lab result is flagged (or turns a different color on their sheet), it draws attention; otherwise, it is often overlooked.
With these staggering predictions, CKD cannot afford to be overlooked or treatment delayed. At this time, which may be a first in our history, CKD has a therapeutic advantage to be tackled early on to prevent progression. This progression frequently occurs under the radar until it is too late for any preventative approach. Our primary providers need our help to identify the early signs of disease initiation and act swiftly to achieve a meaningful delay or halt in progression.

CKD Detection: Simple Tools, Massive Opportunity
One of the most striking aspects of modern CKD care is the contrast between diagnostic simplicity and implementation failure. The condition can be caught early, but therapy is often delayed. This delay is not due to negligence but rather the simple mistake of being overlooked among numerous patient complaints during a visit.
Let’s be honest. I have written about CKD and catching it early for some time. The pressure placed on primary care colleagues is unreasonable. Because of this, I have been advocating for a cultural change in nephrology management. We need to support our primary care colleagues and take the burden of interpretation and therapy implementation off their hands. Nephrology must become more proactive and involved early in patient care. The days of referring only CKD Stage IIIb/IV patients are over. We now have the means to delay and even stop CKD formation and progression.
We need only three readily available tests to screen, diagnose, and stage CKD: serum creatinine to calculate estimated glomerular filtration rate (eGFR) using standardized equations, Cystatin C to estimate eGFR in sarcopenic patients, and urine albumin measurement, typically as an albumin-to-creatinine ratio (Herrington et al., 2025). These simple laboratory values allow us to identify high-risk individuals and distinguish relatively benign CKD presentations from those requiring aggressive intervention.
Despite this diagnostic accessibility, CKD remains chronically underrecognized in primary care documentation. It is frequently absent from problem lists and poorly communicated to patients. The disconnect between what we can detect and what we actually identify in clinical practice represents one of medicine's great implementation gaps. However, we have the means to make significant changes in identification and therapy initiation to help stop CKD formation and progression.
CKD as a Cardiovascular Amplifier
For many patients with CKD, kidney failure is not always the primary threat to their lifespan and health. As Herrington and colleagues emphasize, people with CKD face elevated risks across multiple domains: progressive loss of kidney function, escalation of chronic inflammation contributing to premature cardiovascular events, including myocardial infarction, stroke, heart failure, and accumulating disability long before they would ever require dialysis (Herrington et al., 2025). The sobering reality is that most patients with CKD will die from cardiovascular disease or experience major cardiovascular disability before they ever need kidney replacement therapy (Herrington et al., 2025). Unfortunately, for numerous reasons, CKD amplifies cardiovascular disease due to various pathways involving chronic inflammation and the development of sarcopenia.

This cardiovascular predominance reflects CKD's role as a springboard to worsening overall risk. The disease intertwines traditional cardiovascular risk factors (hypertension, dyslipidemia, diabetes) with CKD-specific mechanisms, including uremic toxin accumulation, anemia, mineral-bone disorders, and chronic systemic inflammation. Understanding CKD primarily as a cardiovascular risk state rather than simply a kidney disease fundamentally reshapes our therapeutic priorities.
It is important to note that the relationship between CKD and cardiovascular disease is bidirectional. In many circumstances, cardiovascular disease may be the main driver. It can influence chronic inflammation, impacting renal function and contributing to CKD and its progression.
The Post-2019 Therapy Revolution
The landscape of CKD therapeutics has transformed since 2019 through landmark randomized controlled trials that have expanded our treatment options beyond renin-angiotensin system (RAS) blockade (Herrington et al., 2025). This transformation can be conceptualized as overlapping waves of kidney-protective therapy.
Renin-angiotensin system inhibitors, including ACE inhibitors and angiotensin receptor blockers, remain foundational. They reduce intraglomerular pressure, lower albuminuria, and decrease the risks of both CKD progression and cardiovascular events in high-risk patients. This established benefit continues to anchor contemporary CKD management.
The second wave arrived with sodium-glucose cotransporter-2 (SGLT2) inhibitors, which revolutionized CKD care by extending benefits beyond glucose control. The DAPA-CKD trial demonstrated that dapagliflozin reduced the primary composite kidney endpoint by approximately 39%, with a remarkably efficient number-needed-to-treat of about 19 over 2.4 years (Heerspink et al., 2020). Similarly, EMPA-KIDNEY showed that empagliflozin reduced the risk of CKD progression or cardiovascular death across a broad CKD population, including many patients without diabetes (The EMPA-KIDNEY Collaborative Group, 2023). These agents slow chronic eGFR decline, reduce kidney failure risk, and decrease heart failure hospitalizations and cardiovascular events—crucially, independent of glycemic effects.

The third wave encompasses non-steroidal mineralocorticoid receptor antagonists (MRAs) and GLP-1 receptor agonists. Finerenone, a selective non-steroidal MRA, demonstrated in the FIDELIO-DKD trial that it could reduce risks of both CKD progression and cardiovascular events in patients with type 2 diabetes and CKD when added to RAS inhibitor therapy, although hyperkalemia risk requires monitoring (Bakris et al., 2020). Most recently, semaglutide entered the kidney-protective arena: the FLOW trial demonstrated that this GLP-1 receptor agonist reduced the risk of major kidney outcomes and cardiovascular death in patients with type 2 diabetes and CKD, prompting regulatory recognition of these kidney benefits in drug labeling (Perkovic et al., 2024; Herrington et al., 2025).
CKD Care Requires Multi-Specialty Coordination
Because CKD functions as a systemic disease with cardiovascular, metabolic, and multi-organ manifestations, affected patients typically interface with primary care, cardiology, endocrinology, nephrology, and geriatrics or palliative care services. The Lancet review argues compellingly that every clinician encountering high-risk patients should maintain CKD awareness, implement consistent screening, and trigger standardized evidence-based interventions. These interventions include blood pressure optimization, RAS inhibition, SGLT2 inhibition, statin therapy, lifestyle modification, and, where appropriate, the addition of non-steroidal MRAs or GLP-1 receptor agonists (Herrington et al., 2025).

This multi-specialty reality demands that we reconceptualize CKD care less as "referral to a nephrology silo" and more as integrated cardio-renal-metabolic management, where expertise is distributed and coordination is paramount.
Implementation: Translating Evidence to Practice
The fundamental message of contemporary CKD management is that we already possess the tools to substantially reduce CKD-related complications; our challenge is deploying them earlier and more consistently across healthcare systems (Herrington et al., 2025). Successful implementation requires systematic attention to five domains.
We must screen appropriate populations: everyone with diabetes, hypertension, established cardiovascular disease, or age 60 years and older should receive periodic eGFR and urine albumin assessment.
We must properly stage and risk-stratify patients using the combined eGFR and albuminuria framework to determine follow-up intensity and therapeutic urgency.
We must initiate kidney- and heart-protective therapies early, with RAS inhibitors and SGLT2 inhibitors forming the foundation in appropriate patients. This should be complemented by statins for atherosclerotic cardiovascular disease prevention and layering in finerenone or GLP-1 receptor agonists where evidence-based and tolerated (Herrington et al., 2025).
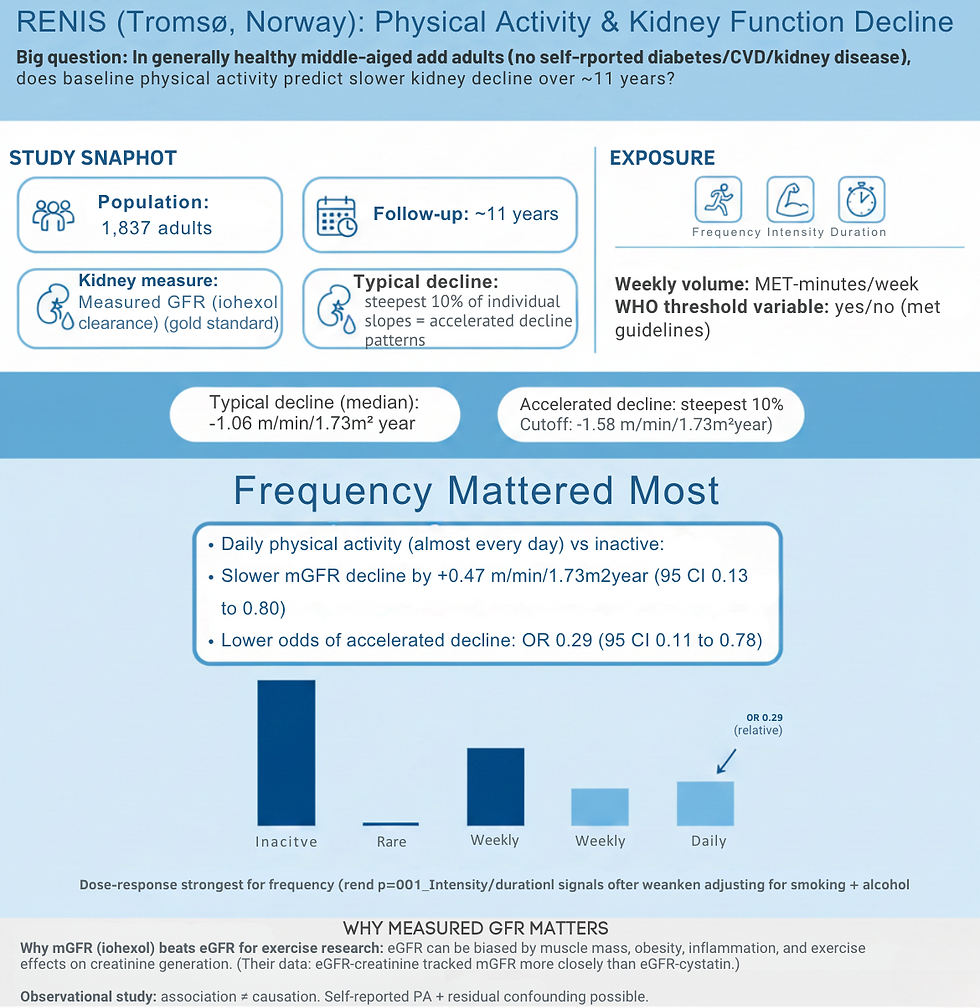
We must address the full spectrum of cardiovascular risk through blood pressure targets, smoking cessation, weight management, exercise prescription, and thoughtful deprescribing when appropriate.
Finally, we must recognize clear nephrology referral triggers, including rapid eGFR decline, heavy albuminuria, resistant hypertension, complex electrolyte disturbances, or advanced G4-G5 CKD requiring preparation for kidney replacement therapy.
Although this final step is based on current recommendations, I challenge all primary providers, specialists, and patients themselves to be more proactive and pull the trigger for nephrology referral earlier. This ensures that proper, goal-directed therapy is implemented early and monitored to give the patient the best possible outcome.
Conclusion
Chronic kidney disease stands at an inflection point. We have moved from offering rudimentary therapeutic options to an evidence-rich era where multiple complementary interventions can meaningfully alter disease trajectories and reduce cardiovascular risk.
The gap between what we know and what we do remains substantial, but it is fundamentally a problem of implementation rather than knowledge. By embracing CKD as a shared responsibility across specialties, systematically applying simple screening tools, and deploying our expanding therapeutic arsenal early and comprehensively, we have an opportunity to bend the curve on what would otherwise become the fifth leading cause of death worldwide within a generation.
References
Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219-2229.
Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436-1446.
Herrington WG, Judge PK, Grams ME, Wanner C. Chronic kidney disease. Lancet. 2025; online ahead of print.
Perkovic V, Tuttle KR, Rossing P, et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N Engl J Med. 2024;391(2):109-121.
The EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117-127.



Comments